Molnupiravir, A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say
Molnupiravir a wide-spectrum antiviral that is currently in phase 23 clinical trials for the treatment of COVID-19 is proposed to inhibit viral replication by a mechanism known as lethal. Molnupiravir ist das Prodrug des synthetischen Nukleosid derivats N4-Hydroxycytidin NHC.
2021 a novel antiviral agent with potent activity against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 the causative agent of coronavirus disease 2019 COVID-19.

Molnupiravir. Tatsächlich zeigt sich Molnupiravir wirksam gegen Sars-CoV-2. As of June 25 2021 SARS-CoV-2. Dienstplanung und Stundenkonten stets im Blick.
Derzeit 2021 wird in klinischen Studien ein möglicher Einsatz bei COVID-19-Patienten geprüft. Molnupiravir an oral antiviral treatment for COVID-19. Molnupiravir MK-4482 is designed to induce viral genome copying errors to prevent the virus from replicating in the human body and evidence to date from clinical trials in patients with COVID-19 suggests that molnupiravir may reduce replication of the SAR-CoV-2 virus.
Molnupiravir is also being studied in a Phase III trial for preventing infection in people exposed to the coronavirus. Wie es das Coronavirus stoppt war bisher unklar. Last updated by Judith Stewart BPharm on July 14 2021.
Based on preliminary clinical trials the compound promises to be highly effective against Sars-CoV-2. Based on preliminary clinical trials the compound promises to be highly effective against SARS-CoV-2. Molnupiravir FDA Approval Status.
The drug looks enough like some of the natural building blocks that the. Molnupiravir another antiviral drug candidate was originally developed to treat influenza. Der Pharmakonzern Merck meldet positive Ergebnisse einer Studie zu einem neuen Corona-Medikament.
Ad Apotal Onlineapotheke - günstiger gehts kaum. US-Forscher haben ihn an Frettchen getestet. Der antivirale Wirkstoff Molnupiravir ist derzeit als mögliches Covid-19-Medikament im Gespräch.
Molnupiravir demonstrated activity in preclinical models of SARS-CoV-2 SARS-CoV-1 and MERS including prophylaxis cure and transmission prevention. Molnupiravir another antiviral drug candidate was originally developed to treat influenza. Dienstplan erstellen und Zeiten erfassen.
Das Medikament Molnupiravir der US-Firma MSD senkt offenbar das Risiko für Krankenhausaufenthalt und Tod durch Covid-19 um die Hälfte. Der antivirale Wirkstoff Molnupiravir könnte eine Ansteckung mit dem Corona-Virus verhindern. Molnupiravir works by confusing SARS-CoV-2s polymerase the enzyme that builds the viral genome during replication.
Molnupiravir is the first oral direct-acting antiviral shown to be highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile. Mit der Smart-TV App holen Sie sich Ihre Apotheke ins Wohnzimmer. Die Einnahme von Molnupiravir habe das Risiko im Krankenhaus behandelt werden zu müssen um die.
In March 2021 the companies reported preliminary results from a Phase IIa trial of molnupiravir for Covid-19. An effective antiviral therapeutic has since been intensively sought. Molnupiravir an Oral Antiviral Treatment for COVID-19.
Molnupiravir increases the frequency of viral RNA mutations. A novel coronavirus originally identified in Wuhan City China was reported to the World Health Organization on 31 December 2019 and the associated disease has subsequently become a worldwide pandemic. Die Firmen MSD und Ridgeback Biotherapeutics haben nach einer Zwischenanalyse angekündigt eine Phase-III-Studie bei nicht hospitalisierten Covid-19-Patienten fortzusetzen.
Eigentlich sollte es ein Mittel gegen Grippe werden. Jetzt haben Göttinger. A recently published article described the safety tolerability and pharmacokinetic profile of molnupiravir Painter et al.
Merck Molnupiravir EIDD-2801MK-4482 is an investigational orally bioavailable form of a potent ribonucleoside analog in development for the treatment of COVID-19. Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19. Molnupiravir an Oral Antiviral Treatment for COVID-19 medRxiv.
Molnupiravir ist ein experimentelles Virustatikum das ursprünglich zur Behandlung von Infektionen durch Influenza- und Coronaviren entwickelt wurde. So geht Personalplanung heute. Merck officials said it is unclear how long the FDA review will take.
This treatment is being evaluated in an ongoing Phase 3 trial for its potential to reduce the risk of hospitalization or.

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say
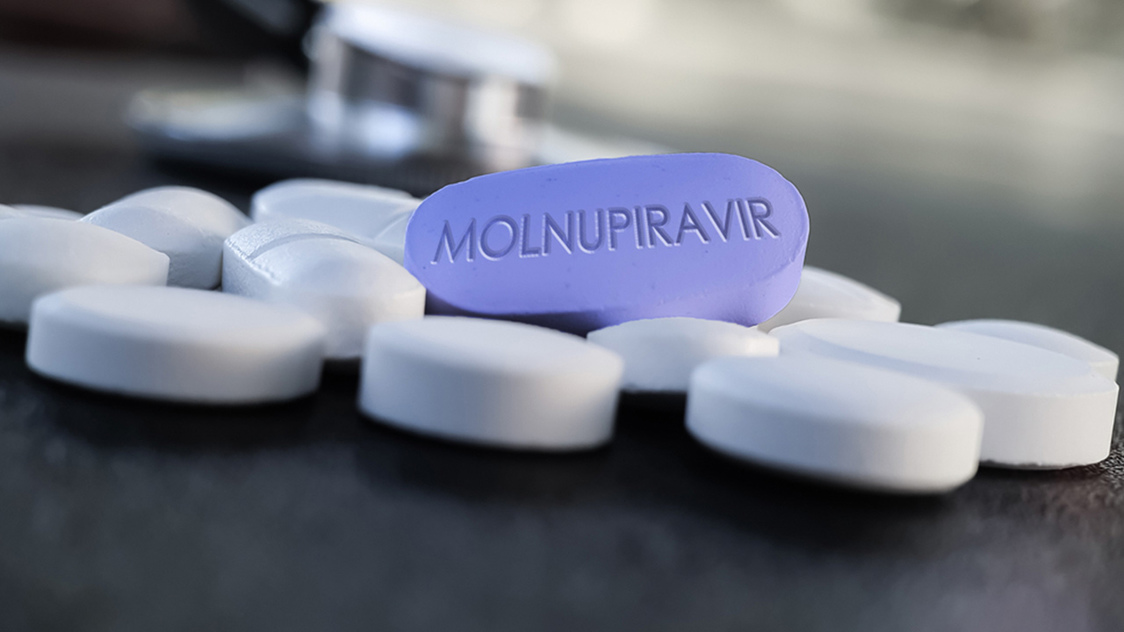
Lung Center Qmmc Call For Participants In Molnupiravir Trials Vs Covid 19

Weniger Schwere Krankheitsverlaufe Corona Medikament Weckt Hoffnung Tagesschau De

Wie Das Potenzielle Coronamedikament Molnupiravir Wirkt

Neue Studiendaten Molnupiravir Schutzt Mause Vor Corona Infektion Und Cov Pz Pharmazeutische Zeitung
Covid 19 Drug Molnupiravir Likely To Be Effective Against Known Variants Merck

Covid Molnupiravir Drugmaker Merck Seeks Marketing Approval Sortiraparis Com





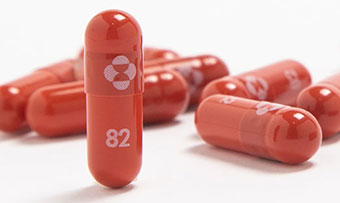
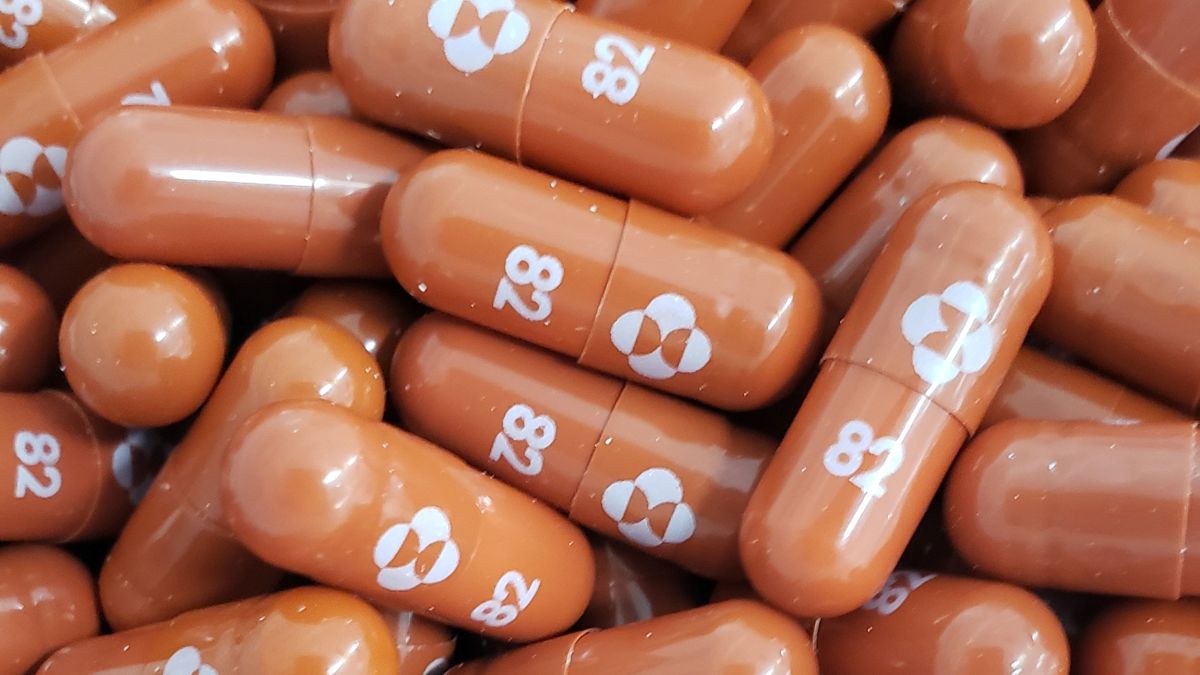






Posting Komentar
Posting Komentar